Pathologic myopia is the leading cause of visual impairment and low vision worldwide.(1-3) The condition refers to degenerative changes in the sclera, choroid, and retinal pigment epithelium (RPE) induced by abnormal axial length elongation in eyes with high myopia. It represents a subgroup of myopia and affects up to 3% of the population.(4) High myopia is defined as a refractive error of at least -6.00D or an axial length of 26.5mm or more.(4) The prevalence of pathologic myopia-related visual impairment has been reported as 0.1%-0.5% in European studies and 0.2% to 1.4% in Asian studies.(5)
Myopic choroidal neovascularisation
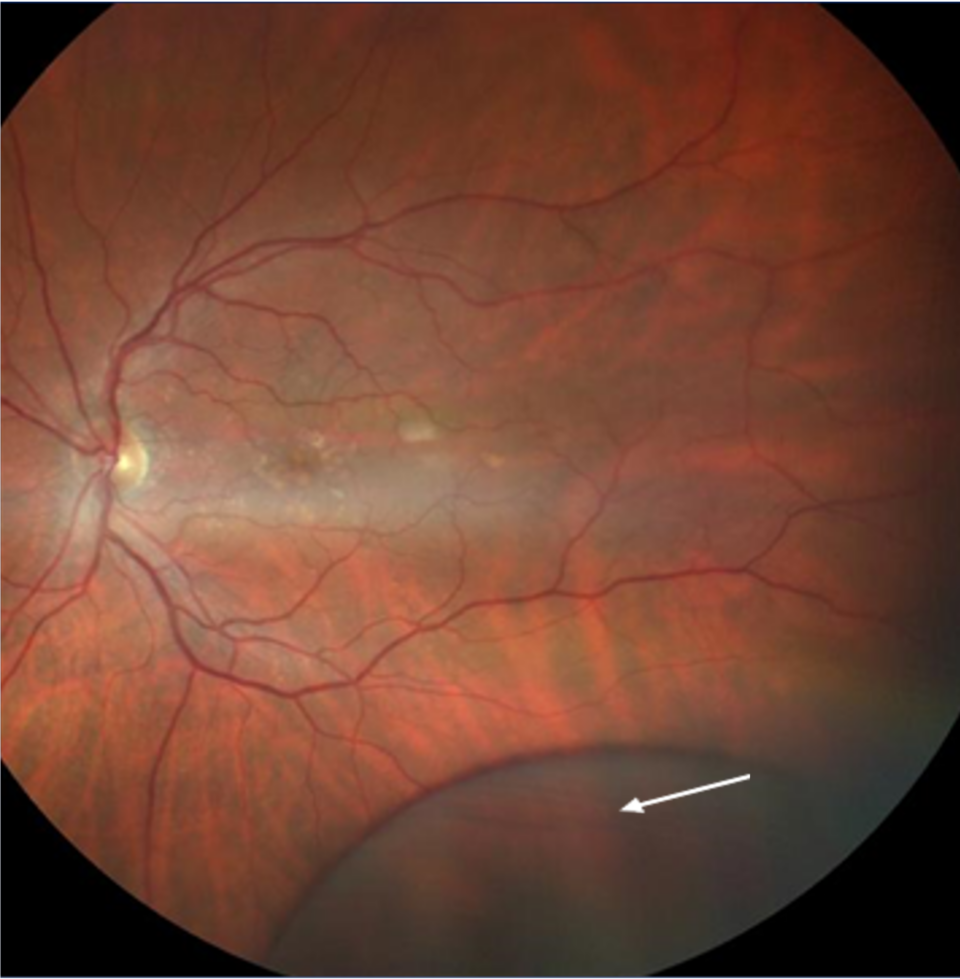
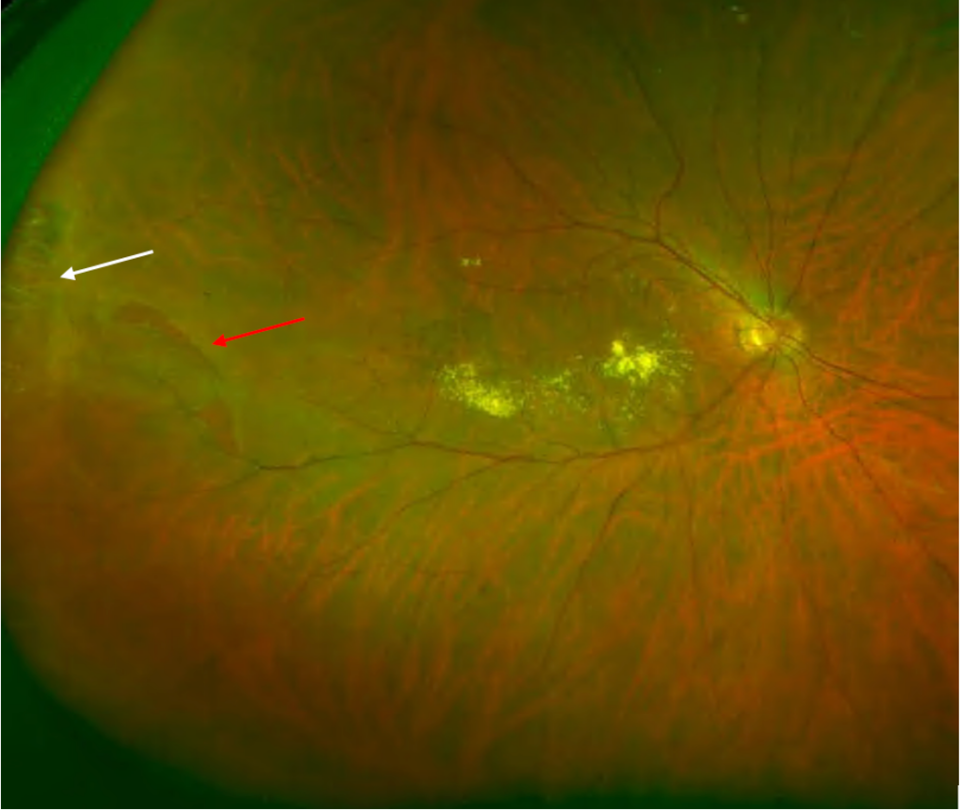
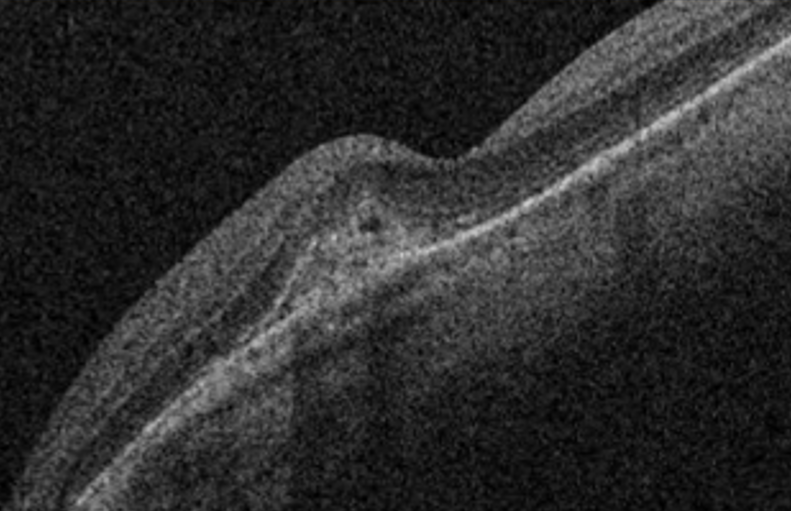
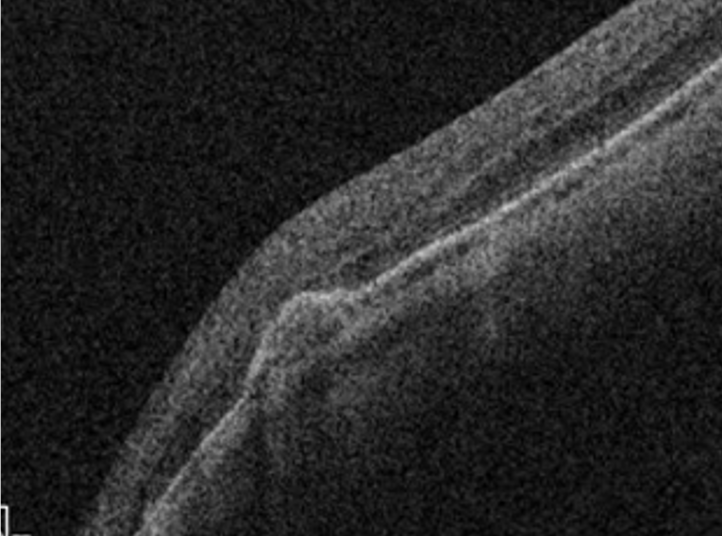
There are various clinical manifestations of myopic maculopathy (macular changes due to myopia): diffuse chorioretinal atrophy, patchy chorioretinal atrophy, myopic CNV, lacquer cracks, and atrophy secondary to CNV. Myopic CNV is one of the most severe complications of myopia, regardless of the axial length. It has been reported in 5-11% of patients with pathologic myopia. Most patients develop CNV before the age of 50.(6) Approximately, there is a risk of 35% of developing CNV in the fellow eye.(7) It is presumed that excessive elongation of the globe and posterior pole causes mechanical stress and retinal damage.(8) Another theory presupposes that CNV results from choroidal hypoperfusion secondary to choroidal thinning in myopic patients.(9)
Retinal peripheral degenerations
Retinal degenerations are common lesions involving the peripheral retina, and most of them are clinically insignificant.(10) Those lesions are classified according to several criteria:
- Location: equatorial, peripheral, or combined
- Pathomorphology: trophic, atrophic, tractional, or combined
- Depth of changes: vitreoretinal, intraretinal, chorioretinal
- The risk for retinal detachment
- Prognosis: stationary or progressive.(11)
For clinical purposes, the risk for retinal detachment is the most important criterion when we consider peripheral retinal degenerations. Lattice degeneration, degenerative retinoschisis, and cystic retinal tufts can result in a rhegmatogenous retinal detachment.(12) Peripheral retinal changes are often present in myopic eyes.(12)
According to depth, we classify the peripheral retinal degenerations as below:
- Vitreoretinal degenerations: lattice degeneration, snail-track degeneration, retinal tufts, and peripheral retinal breaks.
- Intraretinal degenerations: senile retinoschisis, white-without-pressure, dark-without-pressure, peripheral cystoid degeneration, snowflake degeneration, and pearl degeneration.
- Chorioretinal degenerations: paving stone degeneration and peripheral retinal drusen.