Conclusion
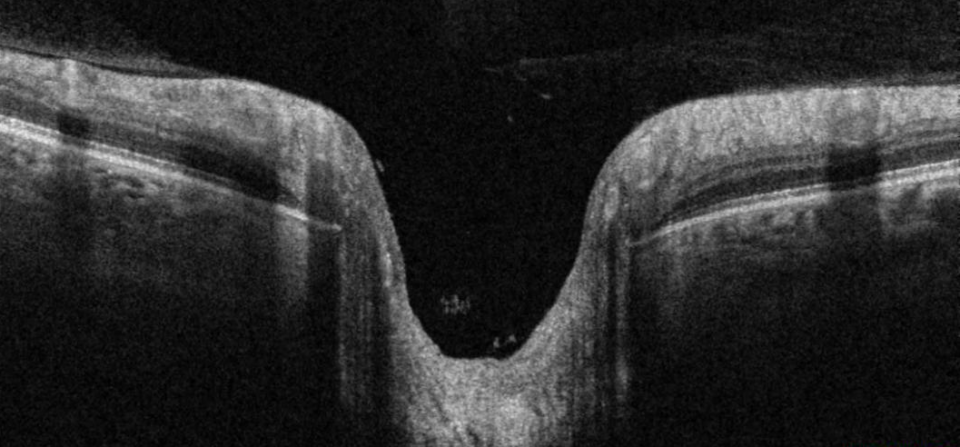
We are all very familiar with the signs of primary open-angle glaucoma (POAG). But if you suspect glaucomatous optic neuropathy, don’t automatically assume that it is caused by the most common open-angle form. A quick check of the angle using either the AS-OCT or the van Herrick method can help identify patients who may be suffering from chronic closed-angle glaucoma. Generally, the condition can be successfully treated by removing the crystalline lens, but an important takeaway is that chronic closed-angle glaucoma is much more common than many optometrists realise.
Secondary glaucoma is rare, but you should nonetheless rule it out in patients with raised intraocular pressure. A careful history, combined with clinical tests including slit lamp, fundus exam and OCT) should be sufficient to rule out conditions that can cause a secondary rise in IOP, i.e., secondary glaucoma. Conversely, patients suffering from conditions such as uveitis, Pseudoexfoliation syndrome, pigment dispersion syndrome or retinal vein occlusion that can lead to secondary glaucoma should have their intraocular pressure checked, regardless of age.
And finally, while many of us struggle to remember the numerous different layers of the retina, an OCT scan provides a rapid, automated analysis of the retinal layers affected by glaucoma (ganglion cell layer and retinal nerve fibre layer) without needing to get bogged down in the details of different layers. These measurements, which can be compared with reference data and/or monitored over time, provide additional information to complement optic nerve head findings that are infamously subjective. OCT metrics can lead to earlier detection of glaucomatous damage associated with asymptomatic glaucoma – i.e., primary open-angle glaucoma and chronic closed-angle glaucoma.